Zoladex® is the only ready-to-use, biodegradable GnRH* agonist implant1-4
The Zoladex SafeSystem® is designed to provide consistent, reliable delivery of Zoladex1
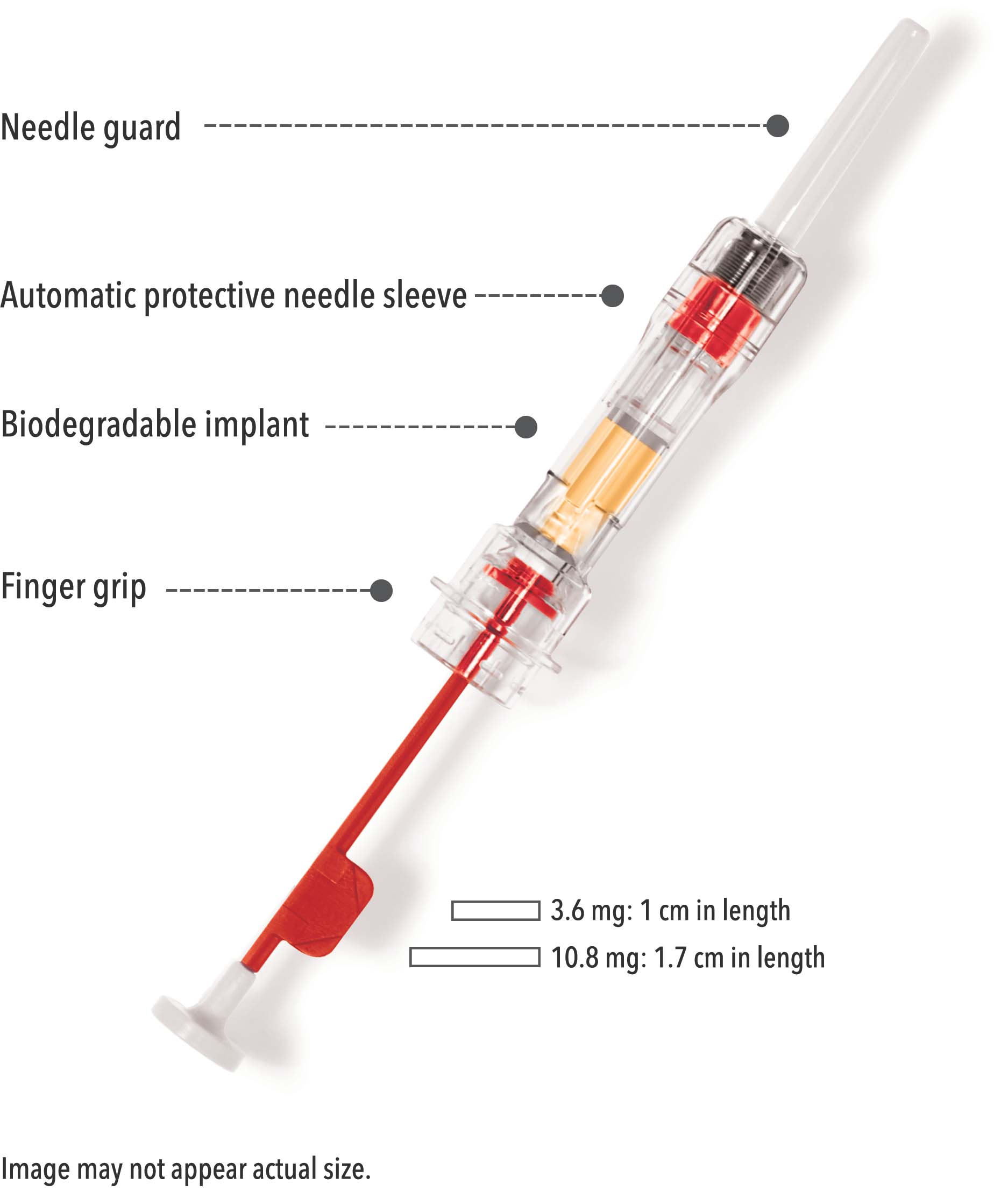
Zoladex features:
- A sterile, siliconized, triple-beveled hypodermic needle with easy-glide SafeSystem
- No assembly or mixing required
- Implant provides consistent, reliable delivery of medication
- Each syringe is provided with a convenience pack (contains gauze, alcohol wipe, and bandage)
-
Designed with a protective needle sleeve and guard to reduce needlestick injuries6
- Zoladex SafeSystem® needle is automatically covered upon withdrawal
Zoladex helps meet the needs of nurses7,8
In a study with nurses, it took an average of 1.70 minutes to prepare and deliver Zoladex7
- Randomized crossover study with 82 nurses timed in the administration (preparation and delivery) of the implant system used to administer Zoladex and the vial system used to administer leuprolide acetate. Preferences and perceptions of the ease of use and relative safety of the 2 injection systems were also assessed.7

The majority of patients reported minimal pain (VAS† <10 mm) from the Zoladex injection8
In a study with patients, there was no significant difference between the pain levels experienced from injections of Zoladex compared to injections of intramuscular leuprolide acetate8
- A total of 50 patients were blindfolded and administered either Zoladex or leuprolide acetate into the anterior abdominal wall. Each group (24 Zoladex and 26 leuprolide acetate) received 2 injections 4 weeks apart. Following each injection, patients were asked to record the pain of injection on a visual analogue scale ranging from 0 mm (no discomfort) to 100 mm (maximal discomfort).8
- †VAS: visual analogue scale.
Zoladex step-by-step administration guide
PREPARATION: No refrigeration or premixing is required with Zoladex—the siliconized hypodermic needle with easy-glide SafeSystem comes ready to administer. You can also download this helpful guide for your office.
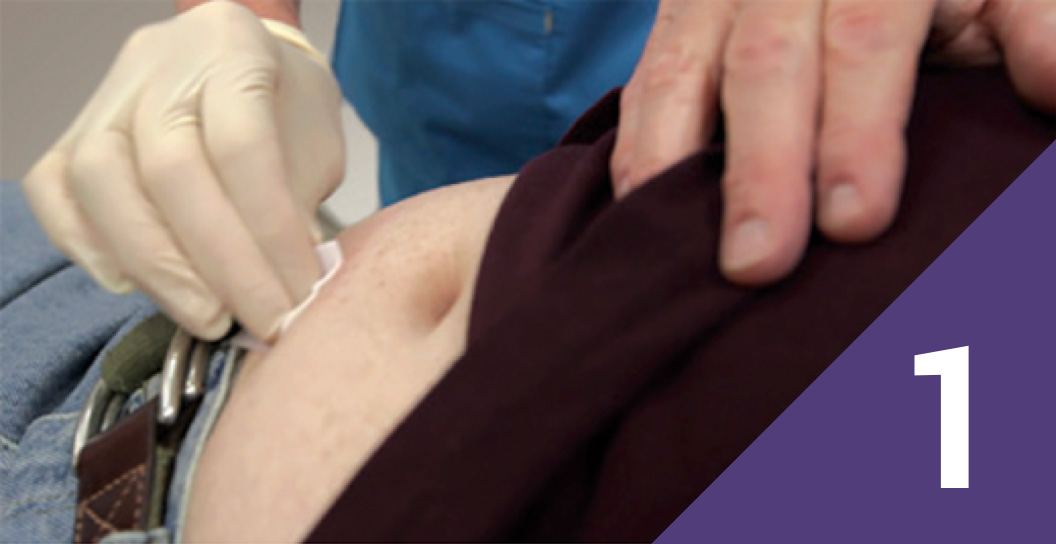
Clean the injection-site area
Clean an area of the anterior abdominal wall (below the navel) for injection using an alcohol swab.
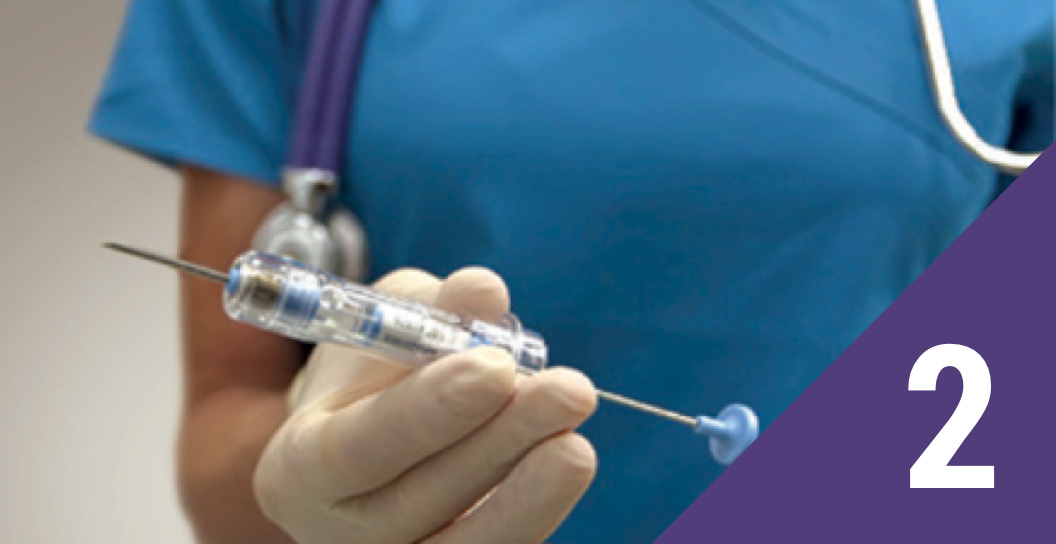
Inspect the syringe
Inspect the foil pouch and syringe for damage. Remove the syringe from the pouch and hold it at a slight angle to make sure part of the implant is visible. Remove the plastic safety tab and needle cover.
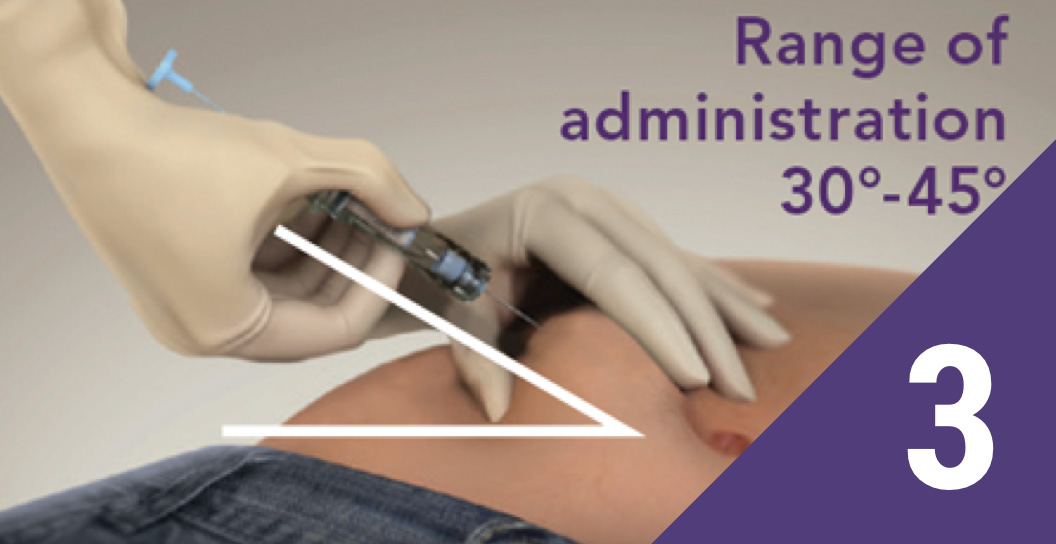
Pinch the skin
Pinch the patient’s skin using aseptic technique at the prepared injection site, and hold the needle with the bevel facing up at an injection angle of 30°-45°.
Note: Extra care should be taken with patients with low BMI and/or patients receiving a full dose of anticoagulation.
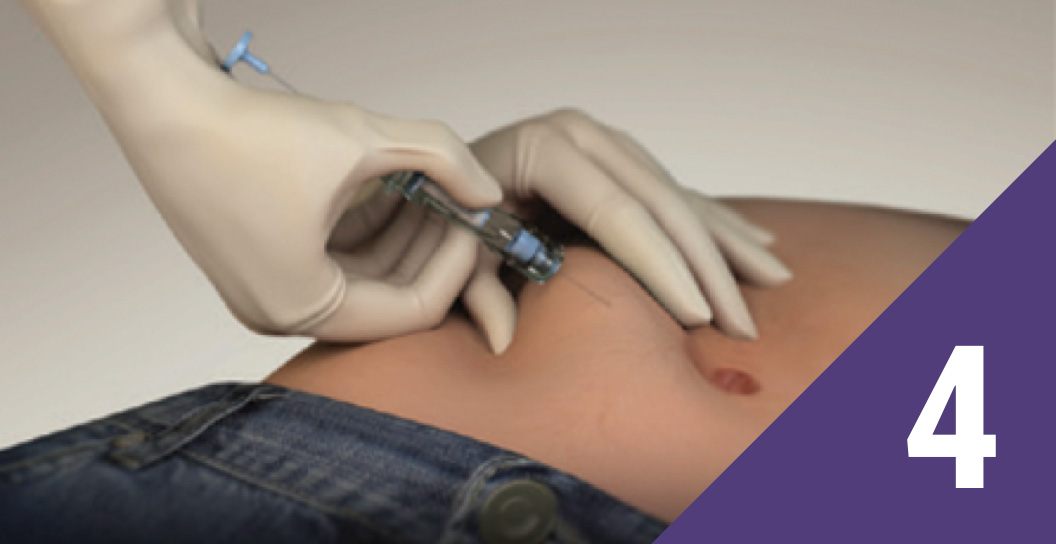
Insert the needle
Insert the needle, with the bevel facing up, until the protective sleeve touches the patient’s skin. Take care not to penetrate the muscle or peritoneum.
Note: If the needle penetrates a large blood vessel, blood will immediately be seen in the syringe chamber. If this occurs, withdraw the needle and inject a new syringe at a new location. Monitor patients for signs of abdominal hemorrhage.
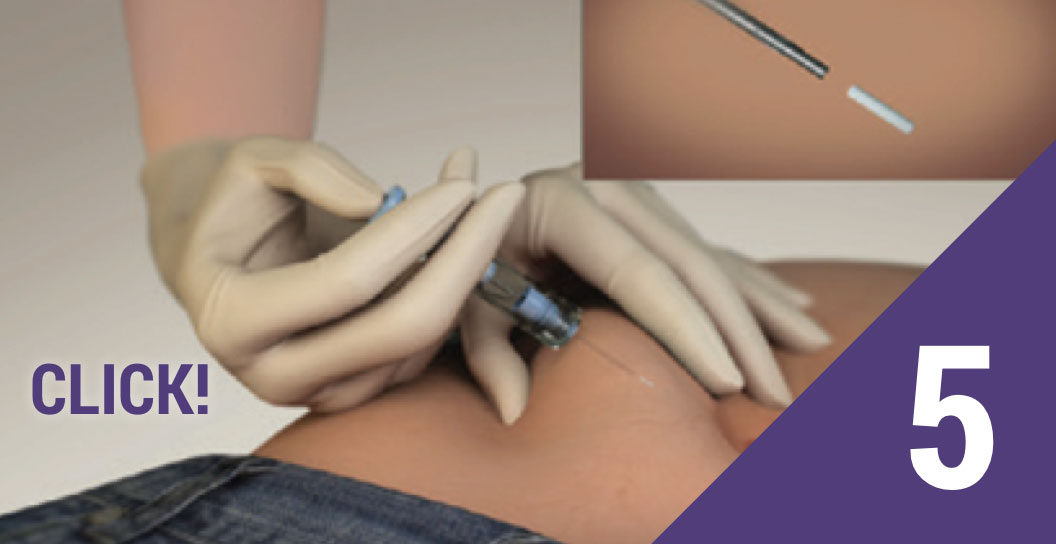
Depress the plunger
Depress the plunger until you hear a “CLICK.” The click ensures the SafeSystem has been activated and the implant has been deposited in the correct location.
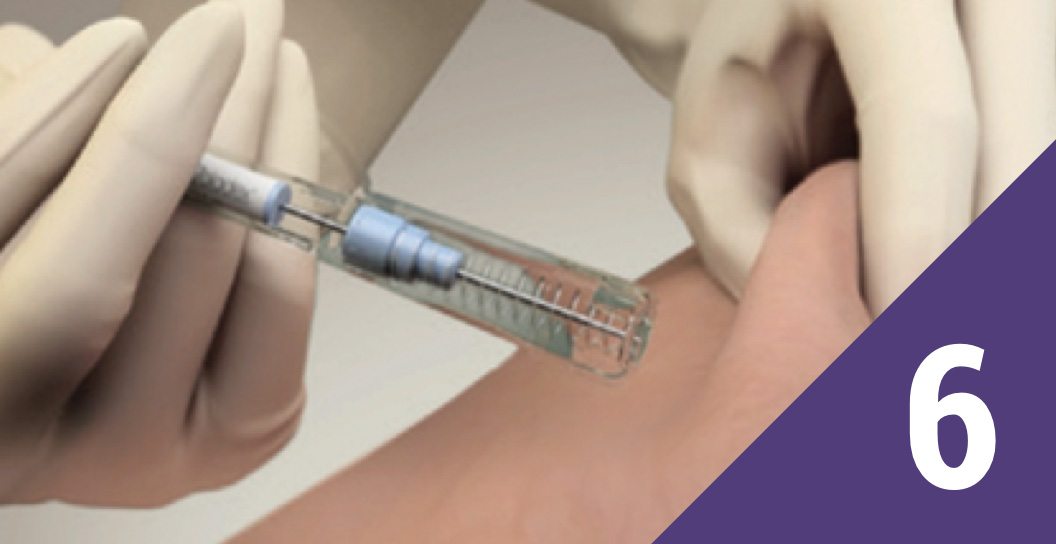
Withdraw the needle
Withdraw the needle and allow the protective sleeve to slide and cover the needle; dispose in an approved sharps container.
Watch this helpful video for more information on how to administer Zoladex
Additional support services such as reimbursement information, patient support, and in-office injection training are available by calling 1-844-ZOLADEX (1-844-965-2339).
*For eligible commercially insured patients, card carries a maximum benefit of $2,000 per calendar year. Eligible cash-paying patients will receive up to $500 off each 1-month supply. You are not eligible if prescriptions are paid by any state or other federally funded programs, including, but not limited to Medicare or Medicaid, Medigap, VA or DOD or TRICARE, or where prohibited by law.
- ZOLADEX® (goserelin implant) 3.6 mg [prescribing information]. Lake Forest, IL: TerSera Therapeutics LLC; 2020.
- LUPRON DEPOT® (leuprolide acetate for depot suspension) [prescribing information]. North Chicago, IL: AbbVie Inc.; 2021.
- ELIGARD® (leuprolide acetate) [prescribing information]. Fort Collins, CO: Tolmar Pharmaceuticals Inc.; 2019.
- TRELSTAR® (triptorelin pamoate for injectable suspension) [prescribing information]. Irvine, CA: Allergan, Inc.; 2020.
- Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Breast Cancer V.3.2018. © National Comprehensive Cancer Network, Inc 2018. All rights reserved. Accessed December 28, 2018. To view the most recent and complete version of the guidelines, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use, or application and disclaims any responsibility for their application or use in any way.
- Moser MA. Engineering out needle stick injuries (safety devices). The Safe Angle. Summer 2004;5-7.
- Morgan G, Cooley C. Injection systems for two luteinising hormone-releasing hormone agonists: a comparative assessment of administration times and nurses’ perceptions. Eur J Oncol Nurs. 2005;9:334-340.
- Montgomery BS, Borwell JP, Higgins DM. Does needle size matter? Patient experience of luteinising hormone-releasing hormone analogue injection. Prostate Cancer Prostatic Dis. 2005;8:66-68.
- Taylor CW, Green S, Dalton WS, et al. Multicenter randomized clinical trial of goserelin versus surgical ovariectomy in premenopausal patients with receptor-positive metastatic breast cancer: an intergroup study. J Clin Oncol. 1998;16:994-999.
- De Vos FY, van Laarhoven HW, Laven JS, et al. Menopausal status and adjuvant hormonal therapy for breast cancer patients: a practical guideline. Crit Rev Oncol Hematol. 2012;84:252-260.
- Rossi L, Pagani O. Adjuvant endocrine therapy in breast cancer: evolving paradigms in premenopausal women. Curr Treat Options Oncol. 2017;18:28. doi:10.1007/s11864-017-0473-1.
Important Safety Information about ZOLADEX
Anaphylactic reactions to ZOLADEX have been reported in the medical literature. ZOLADEX is contraindicated in patients with a known hypersensitivity to GnRH, GnRH agonist analogues, or any of the components in ZOLADEX
ZOLADEX is contraindicated during pregnancy unless used for palliative treatment of advanced breast cancer. ZOLADEX can cause fetal harm when administered to a pregnant woman. If used during pregnancy, the patient should be apprised of the potential hazard to the fetus. There is an increased risk for pregnancy loss due to expected hormonal changes that occur with ZOLADEX treatment. ZOLADEX should not be given to women with undiagnosed abnormal vaginal bleeding
Pregnancy must be excluded for use in benign gynecological conditions. Women should be advised against becoming pregnant while taking ZOLADEX. Effective nonhormonal contraception must be used by all premenopausal women during ZOLADEX therapy and for 12 weeks following discontinuation of therapy
Transient worsening of tumor symptoms, or the occurrence of additional signs and symptoms of breast cancer, may occasionally develop during the first few weeks of treatment. Some patients may experience a temporary increase in bone pain. Monitor patients at risk for complications of tumor flare
Hyperglycemia and an increased risk of developing diabetes or worsening of glycemic control in patients with diabetes have been reported in men receiving GnRH agonists like ZOLADEX. Monitor blood glucose levels and glycosylated hemoglobin (HbA1c) periodically and manage according to current clinical practice
Increased risk of developing myocardial infarction, sudden cardiac death and stroke has been reported in association with use of GnRH agonists like ZOLADEX in men. Patients receiving a GnRH agonist should be monitored for symptoms and signs suggestive of development of cardiovascular disease and be managed according to current clinical practice
Hypercalcemia has been reported in some prostate and breast cancer patients with bone metastases after starting treatment with ZOLADEX. If hypercalcemia does occur, appropriate treatment measures should be initiated
Hypersensitivity, antibody formation and acute anaphylactic reactions have been reported with GnRH agonist analogues
ZOLADEX may cause an increase in cervical resistance. Therefore, caution is recommended when dilating the cervix for endometrial ablation
GnRH agonists may prolong the QT/QTc interval. Providers should consider whether the benefits of androgen deprivation therapy outweigh the potential risks in patients with congenital long QT syndrome, congestive heart failure, frequent electrolyte abnormalities, and in patients taking drugs known to prolong the QT interval. Electrolyte abnormalities should be corrected. Consider periodic monitoring of electrocardiograms and electrolytes
Injection site injury and vascular injury including pain, hematoma, hemorrhage and hemorrhagic shock, requiring blood transfusions and surgical intervention, have been reported with ZOLADEX. Extra care should be taken when administering ZOLADEX to patients with low BMI and/or to patients receiving full dose anticoagulation
Treatment with ZOLADEX may be associated with a reduction in bone mineral density over the course of treatment. Data suggest a possibility of partial reversibility. In women, current available data suggest that recovery of bone loss occurs on cessation of therapy in the majority of patients
In women the most frequently reported adverse reactions were related to hypoestrogenism. The adverse reaction profile was similar for women treated for breast cancer, dysfunctional uterine bleeding, and endometriosis
The most commonly reported adverse reactions with ZOLADEX in clinical trials for endometriosis were: hot flashes (96%), vaginitis (75%), headache (75%), decreased libido (61%), emotional lability (60%), depression (54%), sweating (45%), acne (42%), breast atrophy (33%), seborrhea (26%), and peripheral edema (21%)
The most commonly reported adverse reactions with ZOLADEX in clinical trials for endometrial thinning were: vasodilation/hot flashes (57%), headache (32%), sweating (16%), and abdominal pain (11%)
The most commonly reported adverse reactions with ZOLADEX in breast cancer clinical trials were hot flashes (70%), decreased libido (47.7%), tumor flare (23%), nausea (11%), edema (5%), and malaise/fatigue/lethargy (5%). Injection site reactions were reported in less than 1% of patients
The most commonly observed adverse reactions during ZOLADEX treatment for prostatic carcinoma were due to the expected physiological effects from decreased testosterone levels. The most common adverse reactions (incidence of >5% in prostate clinical trials) were:
For ZOLADEX 3.6-mg: Hot flashes (62%), sexual dysfunction (21%), decreased erections (18%), lower urinary tract symptoms (13%), lethargy (8%), pain (worsened in the first 30 days) (8%), edema (7%), upper respiratory infection (7%), rash (6%), and sweating (6%)
For ZOLADEX 10.8-mg: Hot flashes (64%), pain (general) (14%), gynecomastia (8%), pelvic pain (6%), and bone pain (6%)
In the locally advanced carcinoma of the prostate clinical trial, additional adverse event data were collected for the combination therapy with radiation group during both the hormonal treatment and hormonal treatment plus radiation phases of this study. Adverse experiences (incidence >5%) in both phases of this study were hot flashes (46%), diarrhea (40%), nausea (9%), and skin rash (8%). Treatment with ZOLADEX and flutamide did not add substantially to the toxicity of radiation treatment alone
Indications
ZOLADEX 3.6-mg and ZOLADEX 10.8-mg
Management of locally confined Stage T2b-T4 (Stage B2-C) carcinoma of the prostate in combination with flutamide. Treatment with ZOLADEX and flutamide should start 8 weeks prior to initiating radiation therapy and continue during radiation therapy.
Palliative treatment of advanced carcinoma of the prostate.
ZOLADEX 3.6-mg
Management of endometriosis, including pain relief and reduction of endometriotic lesions for the duration of therapy. Experience with ZOLADEX for the management of endometriosis has been limited to women 18 years of age and older treated for 6 months.
Use as an endometrial-thinning agent prior to endometrial ablation for dysfunctional uterine bleeding.
Palliative treatment of advanced breast cancer in pre- and perimenopausal women.
To report suspected adverse reactions, contact the FDA at 1-800-FDA-1088 or www.FDA.gov/medwatch. You may also contact TerSera Therapeutics at 1-844-334-4035 or medicalinformation@tersera.com.